Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) is a powerful analytical technique widely used for the determination of elemental concentrations in various matrices. It offers advantages like high sensitivity, wide linear range, and the ability to analyze multiple elements simultaneously. However, the accuracy and reliability of ICP-OES analysis heavily rely on proper sample preparation.
This article explores the significance of sample preparation in ICP-OES, discusses common sample types and their preparation techniques, and highlights crucial considerations for achieving optimal results.

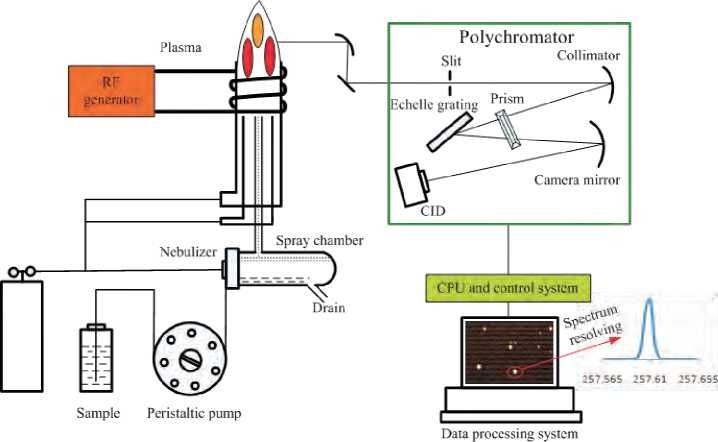
Overview of ICP-OES Analysis
ICP-OES utilizes an inductively coupled plasma (ICP) source to generate highly excited atoms from the sample. These excited atoms emit light at specific wavelengths characteristic of their element. The intensity of this emitted light is then measured by a detector and used to quantify the elemental concentration in the sample.
Since ICP-OES analyzes samples in a liquid form, proper sample preparation is crucial. It ensures the sample is completely dissolved, eliminates interferences from the matrix, and provides a homogenous solution for analysis.
Common Sample Types and Preparation Techniques
In ICP-OES analysis, various sample types are encountered, ranging from environmental samples like water and soil to biological specimens and industrial materials. Each sample type necessitates specific preparation techniques to ensure optimal results.
Different Sample Types Encountered in ICP-OES Analysis
Here are some common sample types encountered in ICP-OES analysis:
- Liquid Samples: These are the most common sample types analyzed using ICP-OES. Liquid samples can include water, acids, solutions, digests, and extracts from various sources such as environmental samples, biological samples, industrial process samples, etc.
- Solid Samples: Solid samples can be analyzed using ICP-OES after digestion or dissolution. This involves converting the solid sample into a liquid form that can be introduced into the ICP-OES instrument. Solid samples may include soils, sediments, ores, metals, ceramics, polymers, and biological tissues.
- Powdered Samples: Some samples come in powder form and may require special sample preparation techniques such as dissolution or fusion before analysis. Examples include powdered metals, minerals, pharmaceuticals, and powdered food products.
- Gaseous Samples: While less common, ICP-OES can be used to analyze gaseous samples by dissolving them into a suitable liquid matrix. This is often done for trace gas analysis or analysis of volatile organic compounds.
Common Sample Preparation Techniques
Sample preparation is a crucial step in ICP-OES analysis to ensure accurate and reliable results. Common sample preparation techniques include:
1. Sample Digestion:
- Acid Digestion: Samples are treated with acids (e.g., nitric acid, hydrochloric acid) to dissolve solid materials and convert analytes into solution.
- Microwave Digestion: Accelerated digestion using microwave energy to break down organic and inorganic materials.
- Fusion Digestion: Suitable for refractory materials where samples are fused with fluxing agents at high temperatures to dissolve analytes.

2. Dilution and Filtration:
- Dilution: Samples with high analyte concentrations are diluted with suitable solvents to bring them within the calibration range of the instrument.
- Filtration: Removal of particulate matter or undissolved solids from the sample solution using filters to prevent blockage of the instrument’s nebulizer or sample introduction system.
3. Solid Phase Extraction (SPE):
Utilization of solid-phase extraction cartridges or disks to selectively retain analytes from the sample matrix while unwanted matrix components are washed away. Analytes are then eluted from the solid phase for analysis.
4. Liquid-Liquid Extraction (LLE):
Separation of analytes from the sample matrix by partitioning between two immiscible liquid phases. After extraction, analytes are concentrated into a smaller volume for analysis.
5. Other Pre-concentration Methods:
Various pre-concentration techniques such as evaporation, solvent extraction, and precipitation are employed to concentrate analytes from large sample volumes, enhancing detection limits and sensitivity.
These sample preparation techniques help ensure that samples are in a suitable form for analysis by ICP-OES, enabling accurate and reliable determination of elemental concentrations. The choice of technique depends on factors such as sample matrix, analyte concentration, required detection limits, and instrument specifications.

Considerations of Sample Preparation for ICP-OES
Several factors influence the efficacy of sample preparation in ICP-OES analysis, including:
Factors Influencing Sample Preparation
Several factors influence sample preparation for ICP-OES analysis. These factors are crucial to consider to ensure accurate and reliable results. Some of the key factors include:
- Sample Type and Matrix Composition:
Different sample matrices require specific preparation methods to ensure complete dissolution and minimize matrix effects.
Samples may vary widely in their composition, such as aqueous solutions, solid materials, biological tissues, or complex matrices like soils or sediments.
- Desired Analytical Sensitivity:
The required detection limits for target analytes influence the choice of sample preparation method.
Lower detection limits often necessitate more extensive sample preparation to concentrate analytes or remove interfering matrix components.
- Sample Throughput and Automation:
The number of samples to be analyzed and the desired analysis throughput affect the choice of sample preparation method.
Automated sample preparation systems may be preferred for high-throughput applications to improve efficiency and reduce labor costs.
- Safety Considerations:
Some sample preparation methods involve hazardous chemicals or high temperatures, requiring adherence to safety protocols to protect personnel and the environment.
Proper ventilation, personal protective equipment (PPE), and containment measures should be implemented as necessary.
- Instrument Compatibility:
Sample preparation methods should be compatible with the specific requirements of the ICP-OES instrument, including the sample introduction system and nebulizer.
Compatibility considerations include sample viscosity, particle size, and potential for sample blockage or instrument contamination.
- Matrix Interference:
Matrix interference from sample components can affect analyte quantification in ICP-OES analysis.
Sample preparation techniques should aim to minimize matrix effects through selective separation, dilution, or matrix matching.
- Sample Homogeneity:
Sample homogeneity ensures representative analysis results by minimizing variability within the sample.
Homogenization techniques such as mixing, grinding, or sonication may be necessary for heterogeneous samples.
- Contamination Control:
Contamination from sample containers, reagents, or laboratory equipment can compromise analytical accuracy.
Proper cleaning procedures and use of high-purity materials are essential to minimize contamination during sample preparation.
By considering these factors, analysts can optimize sample preparation protocols to ensure accurate and precise ICP-OES analysis across a wide range of sample types and applications.

Considerations for Specific Sample Types
Considerations for specific sample types in ICP-OES analysis involve tailoring sample preparation methods to address unique challenges associated with different sample matrices. Here are considerations for several common sample types:
1. Environmental Samples:
- Often complex matrices containing high levels of dissolved solids, suspended particulates, and organic matter.
- Acid digestion or microwave digestion may be necessary to dissolve organic and inorganic constituents.
- Filtration or centrifugation may be required to remove particulates before analysis.
- Matrix matching with certified reference materials (CRMs) can help mitigate matrix effects.
2. Biological Samples:
- Biological samples such as blood, urine, or tissue specimens may contain proteins, lipids, and other biomolecules that can interfere with analysis.
- Sample pretreatment methods like protein precipitation or lipid extraction may be necessary to remove interfering substances.
- Digestion with oxidizing acids or enzymatic treatments may be employed to solubilize analytes of interest.
3. Food and Beverage Samples:
- Food and beverage samples may contain high levels of organic compounds, sugars, and salts.
- Sample preparation methods may involve extraction with solvents or dilution to reduce matrix complexity.
- Acid digestion or wet ashing may be used for total elemental analysis, especially for solid samples like grains or spices.
4. Pharmaceutical Samples:
- Pharmaceutical samples may contain active pharmaceutical ingredients (APIs), excipients, and impurities.
- Sample preparation may involve dissolution of solid dosage forms followed by filtration or centrifugation to remove insoluble components.
- Solid-phase extraction (SPE) or liquid-liquid extraction (LLE) may be employed for purification and concentration of analytes.
5. Industrial Samples:
- Industrial samples can encompass a wide range of materials, including metals, alloys, ceramics, and polymers.
- Sample preparation methods may vary depending on the nature of the material and the analytes of interest.
- Fusion digestion or acid digestion followed by dilution may be employed for metals analysis in metallic samples.
- Solvent extraction or dissolution may be used for organic compounds in industrial fluids or products.
6. Geological Samples:
- Geological samples such as rocks, minerals, and soils may contain high levels of silicates, carbonates, and other minerals.
- Sample preparation may involve fusion digestion or acid digestion with strong mineral acids to dissolve refractory minerals.
- Particle size reduction and homogenization techniques may be necessary to ensure sample representativeness.
By considering these specific sample type considerations, analysts can develop tailored sample preparation protocols to achieve accurate and reliable results in ICP-OES analysis.
Conclusion
Sample preparation is a critical aspect of ICP-OES analysis, significantly impacting the accuracy, precision, and reliability of results. By understanding the principles of the technique, common sample types, and appropriate preparation methods, analysts can ensure accurate, reliable, and reproducible elemental determinations. Careful consideration of factors affecting sample preparation, along with specific techniques for different sample matrices, is crucial for achieving optimal results in ICP-OES analysis.






