Atomic Absorption Spectrophotometer (AAS)
Features | How to Choose | Application | Image | Why Choose Drawell | Feedbacks | Other Products | FAQ
Atomic absorption spectroscopy, or AAS, is a technique for measuring the concentrations of metallic elements in different materials. As an analytical technique, it uses electromagnetic wavelengths, coming from a light source. Distinct elements will absorb these wavelengths differently. It gives a picture of what concentrations of a specific element there is in whatever material, or liquid, is being tested.
Atomic Absorption Spectrophotometer also known as Atomic Absorption Spectrometer, is a specific type of spectrophotometer designed for the analysis of the concentration of individual elements in a sample through atomic absorption spectroscopy.
Features of AAS
For multi-element detection, the specially designed 8-lamp holder supports conventional element memory and allows preheating of 1 to 4 lamps simultaneously. This ensures the light source is always on standby, enhancing ease of use.
DW-AA320N

DW-AA320N is a double beam manual flame type; 1 position lamp holder.
DW-AA4530F
DW-AA4530F is a single beam automatic flame type; 8-positions lamp holder.
DW-AA4730FG

DW-AA4730FG is a single beam fully automatic flame and graphite furnace type; 8 position lamp holder.
DW-AFS310

DW-AFS310 is a single beam fully automatic flame type; 2 position lamp holder.
DW-220A/B
DW-220A/B is a single beam fully automatic flame and furnace type; 8 position lamp holder.
How to Choose the AAS Model?
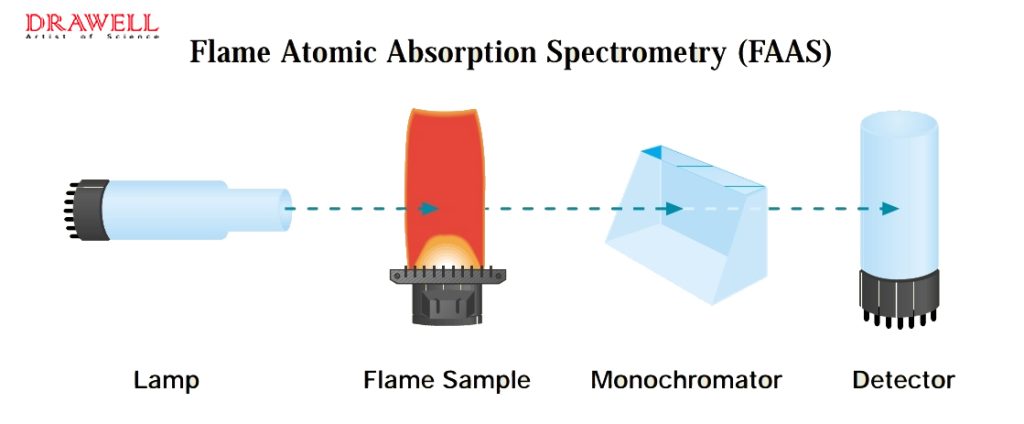
Flame Atomic Absorption Spectrometry (FAAS) uses an air/acetylene or nitrous oxide/acetylene flame to evaporate solvents and dissociate samples into atoms. Light from a hollow cathode lamp, based on the element being measured, passes through the atomic cloud, where the element absorbs the light. The detector measures this absorption to calculate the element’s concentration. FAAS operates at a maximum temperature of 2600°C, effective for elements like alkali metals, heavy metals, and transition metals, but less sensitive for refractory elements like V, Zr, Mo, and B due to temperature limitations.
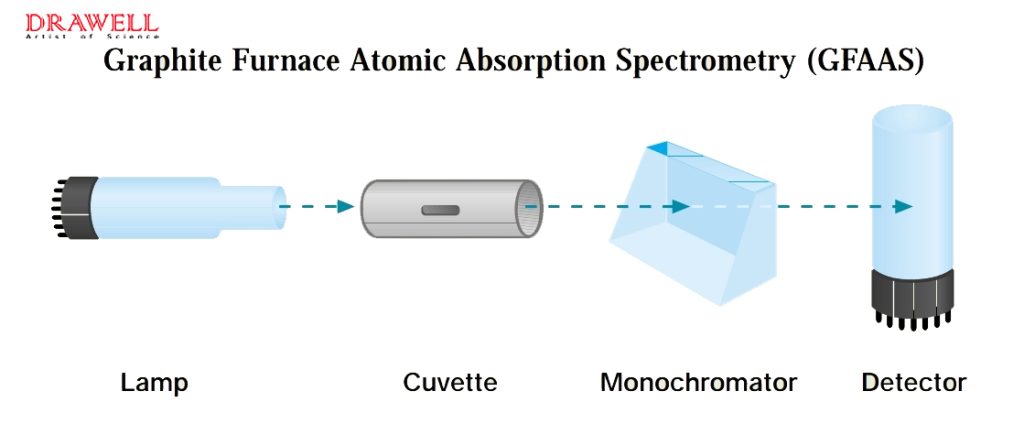
Graphite Furnace Atomic Absorption Spectrometry (GFAAS) operates similarly to FAAS but replaces the flame with an electrically heated graphite tube that can reach up to 3000°C. This increases atom density and residence time, improving detection limits by up to 1000x, achieving sub-ppb sensitivity. However, the performance for refractory elements is still limited due to temperature and graphite cuvette use.
- Flame: All models available
- Graphite furnace: DW-220A, DW-AA320N, DW-AA4530F, DW-AA4730FG
- Hydride generator: Some Special elements like As, Pb, Sn, Se, Hg, Sb, Bi, Ge, Te need to test by AAS with Hydride Generator.
How Does AAS Work?
Principle: Atomic absorption Spectrophotometer (AAS) is based upon the principle that free atoms in the ground state can absorb light of a certain wavelength. Absorption for each element is specific, no other elements absorb this wavelength. It uses a light source that emits a specific wavelength of light, which is then passed through a sample containing the element of interest. The decrease in intensity of the transmitted light is proportional to the concentration of the element.
- Use a light source to emit characteristic spectral radiation of the element to be measured
- This radiation is absorbed by the ground state atoms of the element to be measured in the sample vapor produced by the flame atomizer or graphite furnace atomizer
- Measure the amount of characteristic radiation absorbed
- Calculate the content of the element to be measured based on the functional relationship between the change in light energy and the concentration of the element to be measured (Beer’s law).
Applications of Atomic Absorption Spectrophotometer

AAS for Mining
In the mining industry, atomic absorption spectrometry is used to analyse samples and determine if sites have the potential to be profitable. The technique is used to recover precious metals such as gold and silver, as well as resources such as copper and iron ore.
AAS for Pharmaceuticals
From developing new products to quality control testing, atomic absorption spectrometry is a mainstay in pharmacology laboratories. It can be used to determine if a metal catalyst after a drug has been purified. For example, AAS is used to ensure antibiotics are free from toxic metals such as platinum and palladium before hitting consumer shelves.

AAS for Environmental
The ability to detect miniscule quantities of contaminants and metallic particles has made atomic absorption spectrophotometer a valuable tool used for H2O analysis. It’s used by municipalities to sample drinking water and by environmental scientists to monitor water quality.

AAS for Agriculture
Atomic absorption spectrometry are frequently used in various areas of agricultural research. Typically, they monitor metal contaminants in soil and water, identifying and analyzing potentially harmful elements.

AAS for Forensics
Over the past decade, atomic absorption spectrometry has drastically improved the accuracy of toxicology reports, allowing forensic scientists to detect even the smallest traces of lethal metals such as lead and mercury. The technique is used to analyse muscle and brain tissue, as well as detect gunpowder residue.

AAS for Food & Beverage
Even the smallest traces of heavy metals can cause serious health problems when consumed by human. The food, beverage and health supplements sectors rely on atomic absorption spectrometry to test products and ensure they’re safe for consumption.

AAS for Archaeology
The ability to detect trace elements in artefacts makes atomic absorption spectrometry a useful tool for archaeological analysis. It’s used to gain insight into the elemental composition of artefacts, which can reveal information about age, origins and uses.
Products Display

Different Applications of Spectrophotometer
Choose the spectrometer series that suits you:
Why Choose Us for AAS?
“Multiple suppliers” have always been an issue in the procurement process. Drawell as a one-stop laboratory equipment and scientific instruments supplier, can perfectly solve this problem. In addition to manufacturing our own equipment, we also represent other laboratory equipment. Our product lines are rich and diverse at competitive prices. Provide one-stop service to customers.
User Training – Training by Drawell skilled engineers about installation, debug tests, technical services, etc. It can happen in our factory in China, or at the site in the customers’ country. Cost depends on where and when the training happens.
To discuss the problem and get it resolved, online chats, real-time video calls, and remote guidance. For the after-sales stage, our online technical guidance is free and ready forever.
1 year free official warranty, including repairing quality-damaged parts, and offering replacements of selected parts (shipping cost is extra). 5% of the product price is charged for extending the warranty before the end of the official warranty.
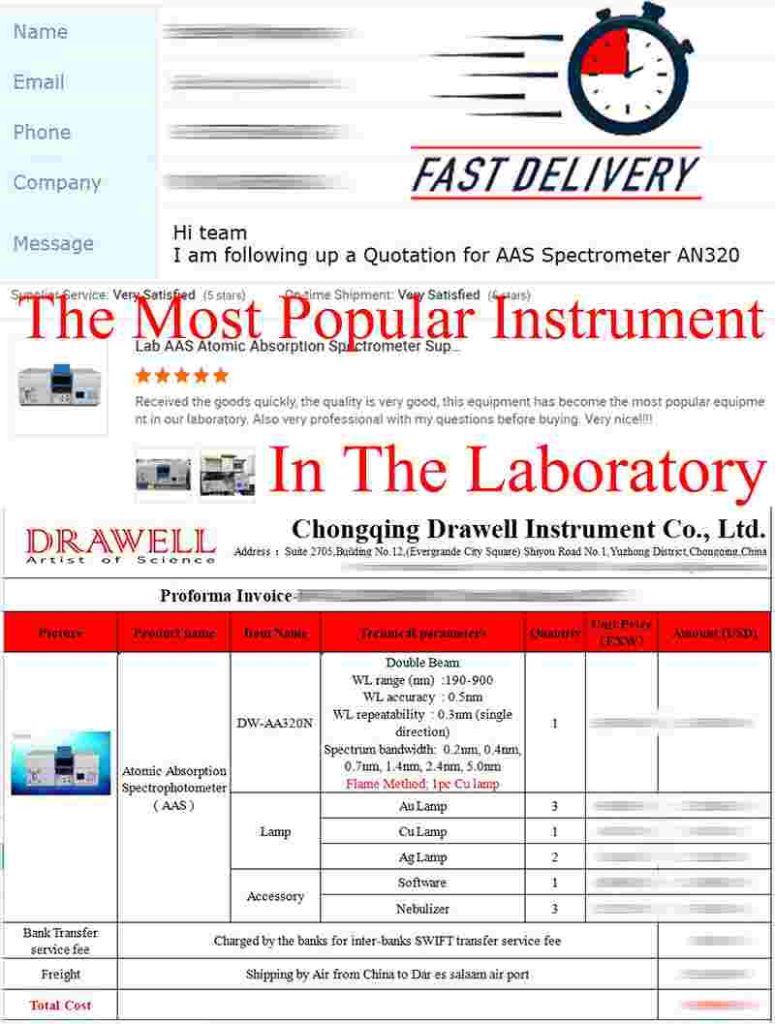
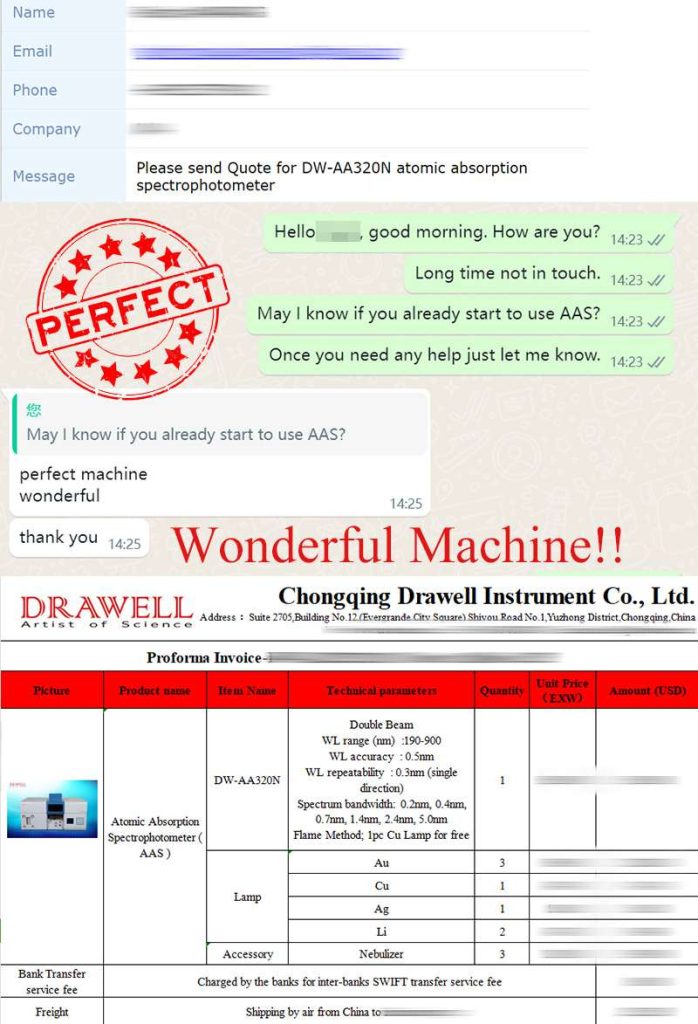
Customer Feedbacks:


Other Spectrophotometers
FAQ
Order Process of AAS