Gas Chromatography (GC) stands as a cornerstone analytical technique, widely employed for separating, identifying, and quantifying volatile and semi-volatile compounds across diverse matrices. Its versatility spans from environmental monitoring and food safety to pharmaceutical quality control and forensic science. Often coupled with Mass Spectrometry (MS) to form GC-MS, it provides unparalleled specificity and sensitivity. However, the sophistication of the GC or GC-MS instrument alone does not guarantee high-quality results.
The adage “garbage in, garbage out” profoundly applies to chromatography, highlighting the critical role of sample preparation for gas chromatography. This initial, often labor-intensive, step is paramount in transforming raw samples into a form suitable for injection into the GC system, directly impacting the accuracy, precision, and reliability of the final data. Here we explore the basic principles, common challenges, and innovative solutions related to gas chromatography sample preparation, aiming to help gas chromatography analysts optimize their analytical workflow and improve their analytical efficiency.

How Sample Preparation Impacts GC Performance
The ultimate goal of sample preparation in GC is to present the analytes of interest to the chromatographic system in a clean, concentrated, and compatible matrix. Without proper sample preparation, analytical challenges such as matrix interference, detector fouling, column degradation, and poor peak resolution become prevalent, leading to unreliable data and increased maintenance costs. A well-prepared sample safeguards the instrument, enhances analytical sensitivity, and ensures the integrity of the data.
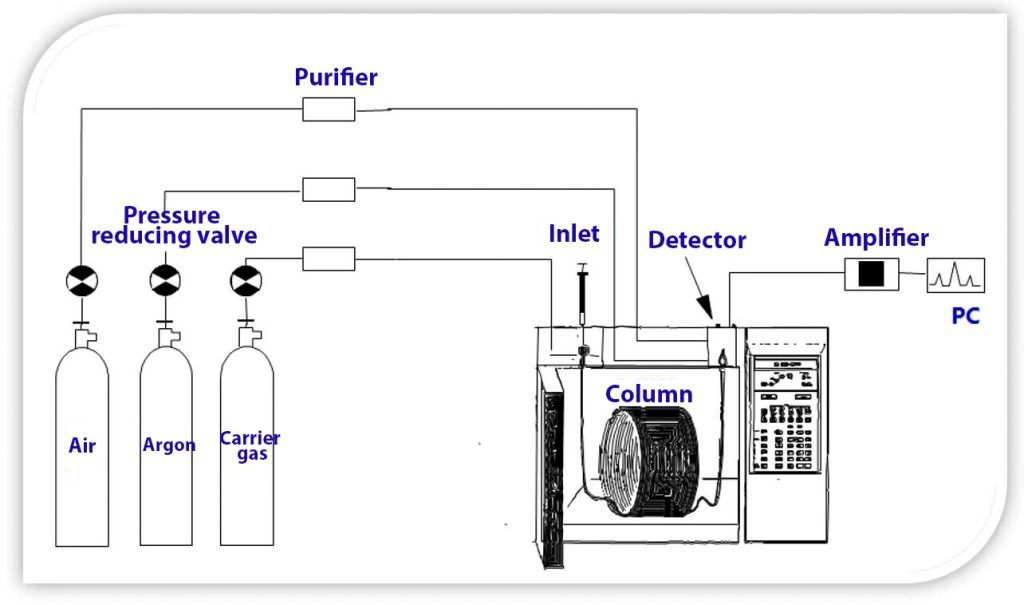
Firstly, proper sample preparation protects the delicate GC system components, particularly the injector port and the analytical column. Raw samples often contain non-volatile matrix components such as salts, proteins, or lipids, which, if injected directly, can precipitate in the hot injector, foul the detector, or irreversibly coat the stationary phase of the column. For instance, injecting a crude biological extract without prior cleanup can lead to rapid column degradation, manifested as increased column bleed, loss of resolution, and shortened column lifetime, necessitating frequent replacement and incurring significant costs. In a study analyzing pesticide residues in food, inadequate sample cleanup led to matrix accumulation in the injector liner and column inlet, resulting in peak tailing and a 50% reduction in column life compared to samples processed with a robust QuEChERS method.
Secondly, sample preparation is crucial for analyte concentration, which is vital when dealing with trace-level compounds. Many environmental and biological samples contain analytes at very low concentrations, often below the direct detection limits of GC. Techniques like solid-phase extraction (SPE) or solid-phase microextraction (SPME) can effectively extract and concentrate analytes from a large sample volume into a smaller, more manageable volume, significantly boosting sensitivity. For example, analyzing volatile organic compounds (VOCs) in water at parts-per-billion (ppb) levels typically requires purge-and-trap concentration, where VOCs are purged from several milliliters of water and concentrated onto an adsorbent trap before thermal desorption into the GC. Without this concentration step, detection at such low levels would be impossible for many GC systems.
Thirdly, efficient sample preparation removes interfering matrix components that might co-elute with the target analytes, obscuring their peaks or causing signal suppression/enhancement in the detector, particularly in GC-MS. These matrix effects can lead to inaccurate quantification. Consider the analysis of drugs in plasma via sample preparation for GC-MS. Plasma is a complex matrix rich in proteins, lipids, and endogenous metabolites. If not adequately removed, these compounds can interfere with the ionization process in the MS, leading to unpredictable signal responses. A properly executed protein precipitation followed by liquid-liquid extraction (LLE) or SPE can effectively isolate the drug, minimizing matrix effects and ensuring accurate quantification.
Finally, proper sample preparation contributes significantly to the reproducibility and accuracy of the analytical results. Consistent and robust sample preparation protocols minimize variability introduced at this stage, leading to tighter quality control and more reliable data. Inconsistent extraction efficiencies or variable matrix removal between samples can lead to significant run-to-run variations, making data interpretation challenging and compromising the validity of the analytical findings.
In a word, the impact of sample preparation on GC performance cannot be overstated. It is a critical determinant of instrument longevity, analytical sensitivity, data quality, and the overall efficiency of the chromatographic workflow. Investing time and resources in optimizing GC sample preparation protocols is an investment in the reliability and validity of your analytical results.

Sample Preparation Techniques for Gas Chromatography
There are many different sample preparation methods for gas chromatography, each targeting specific problems posed by different sample matrices and analyte characteristics. Selecting the appropriate method is critical and requires consideration of factors such as the physical state of the sample, the volatility and concentration of the analytes, and the degree of matrix cleanup required. The following are some common sample preparation techniques:
| Technique | Principle | Advantages | Disadvantages | Typical Applications |
| Dilution | Reducing analyte concentration by adding solvent | Simple, fast, low cost | Reduces sensitivity, doesn’t remove matrix | Highly concentrated samples, non-interfering matrices |
| Filtration | Physical removal of particulate matter | Simple, protects column, prevents clogs | Doesn’t remove dissolved matrix, no concentration | Particulate-laden liquids, general cleanup |
| Liquid-Liquid Extraction (LLE) | Partitioning analytes between two immiscible phases | Effective for diverse analytes, relatively simple | Solvent intensive, emulsion formation, labor-intensive | Environmental pollutants, drugs in biological fluids |
| Solid-Phase Extraction (SPE) | Adsorption/elution of analytes on a solid sorbent | High selectivity, reduced solvent, automation potential | Sorbent selection critical, can be time-consuming | Pesticides in water, pharmaceuticals, food analysis |
| Solid-Phase Microextraction (SPME) | Adsorption of analytes onto a fused silica fiber | Solvent-free, miniaturized, integrates with GC | Limited capacity, fiber degradation, matrix effects | VOCs in water/air, flavors, forensics |
| Stir Bar Sorptive Extraction (SBSE) | Adsorption onto a larger volume sorbent on a stir bar | Higher capacity than SPME, solvent-free | Longer extraction times, specialized equipment | Trace organics in water, flavors, fragrances |
| Headspace (Static/Dynamic) | Equilibration of volatiles between sample and gas phase | Solvent-free, ideal for volatiles, protects GC | Limited to volatiles, can be less sensitive (static) | Residual solvents, blood alcohol, flavors |
| Purge-and-Trap | Dynamic stripping of volatiles with inert gas | Highly sensitive for VOCs, automated | Complex system, high initial cost | VOCs in water/soil, air analysis |
| QuEChERS | Dispersive SPE with salting out | Quick, easy, multi-residue, low solvent | Limited to certain matrices (food), specific analytes | Pesticides in fruits/vegetables |
| Derivatization | Chemical modification to enhance volatility/stability | Enables GC analysis of non-volatile/polar compounds | Adds step, potential for side reactions | Sugars, amino acids, fatty acids |
From simple clean-up to complex extraction, a range of sample preparation techniques for gas chromatography are available, each serving distinct purposes.
- Basic methods like dilution, filtration, and centrifugation offer preliminary cleanup, removing particulates or reducing analyte concentration, though they don’t significantly concentrate analytes or remove dissolved matrix components.
- More advanced approaches include Liquid-Liquid Extraction (LLE), a classical technique that separates analytes based on their solubility in immiscible solvents, though it can be solvent-intensive and prone to emulsion formation, such as when extracting hydrophobic pesticides from water.
- Solid-Phase Extraction (SPE) provides a more refined alternative, using a solid sorbent to selectively retain analytes from a liquid sample, offering enhanced selectivity, reduced solvent use, and greater automation potential, exemplified by using a mixed-mode ion-exchange cartridge to extract polar drugs from urine.
- Solvent-free and miniaturized techniques like Solid-Phase Microextraction (SPME) and Stir Bar Sorptive Extraction (SBSE) extract analytes directly onto a coated fiber or stir bar, respectively, before thermal desorption into the GC, proving ideal for volatile compounds and often used in forensic analysis of ignitable liquid residues.
- For highly volatile compounds, headspace techniques are crucial: Static Headspace (SHS) equilibrates analytes in the gas phase above a sample (e.g., residual solvents in pharmaceuticals), while the more sensitive Dynamic Headspace (Purge-and-Trap) actively strips volatiles onto a trap for subsequent desorption (e.g., VOCs in drinking water).
- Finally, derivatization chemically modifies non-volatile or thermally labile analytes, such as sugars or amino acids, to enhance their volatility and stability for GC analysis.
When performing sample preparation for GC-MS, techniques that ensure excellent cleanup, like SPE, SPME, and Purge-and-Trap, are often preferred to minimize matrix effects and protect the sensitive mass spectrometer. Ultimately, selecting the optimal technique requires balancing desired sensitivity, sample complexity, and throughput.
Common Challenges in GC Sample Preparation and How to Overcome
Although there are many methods for sample preparation in gas chromatography analysis, there are still many challenges that seriously affect the accuracy and reliability of the analysis results. These challenges mainly come from the complexity of actual samples and the delicate nature of trace analysis. Therefore, effectively overcoming these challenges is crucial to obtaining high-quality analysis results.
1. Matrix Effects and Interference
Matrix effects occur when components in the sample interfere with the detection or separation of target analytes. These interferences can cause distorted peaks, reduced sensitivity, or inaccurate quantification. For instance, fatty acids in food samples can overlap with analytes of interest.
How to Overcome:
- Solid-Phase Extraction (SPE): Selectively remove unwanted matrix components while retaining the target analytes.
- Matrix-Matching: Use calibration standards prepared in the same matrix as the sample to account for interference effects.
- Dilution and Filtration: For less complex matrices, diluting the sample or using fine filtration can reduce interference without extensive preparation.
2. Low Analyte Concentration and Sensitivity Limitations
Trace-level analytes can be difficult to detect due to instrument sensitivity limitations, particularly in complex matrices. For example, pesticides in drinking water often occur at extremely low concentrations.
How to Overcome:
- Concentration Techniques: Employ methods like evaporation under vacuum or solid-phase microextraction (SPME) to concentrate analytes before injection.
- Preconcentration Steps: Use techniques such as liquid-liquid extraction (LLE) to enhance analyte detectability.
- Optimization of GC-MS Parameters: For GC-MS, fine-tune ion source and detector settings to improve sensitivity for low-concentration analytes.

3. Sample Volatility and Thermal Degradation
Some analytes are highly volatile or degrade under heat, making them unsuitable for direct GC analysis without prior modification. For instance, thermally labile pharmaceutical compounds may decompose during injection or separation.
How to Overcome:
- Derivatization: Convert sensitive analytes into stable derivatives to improve volatility and thermal stability.
- Low-Temperature Injection: Use cold or programmed-temperature injection to minimize heat exposure during sample introduction.
- Proper Storage: Store samples at low temperatures with minimal exposure to air and light to maintain their integrity.
4. Sample Clean-Up Efficiency and Selectivity
Efficient sample clean-up is crucial to removing unwanted components while retaining analytes of interest. Poor clean-up can lead to fouling of the GC column and inaccurate results.
How to Overcome:
- Selective Adsorption Techniques: Use sorbents tailored to remove specific matrix components while preserving target analytes.
- QuEChERS Method: A quick and versatile approach for cleaning up complex matrices, especially in food and pesticide analysis.
- Optimized Extraction Conditions: Adjust solvent polarity, pH, and extraction time to improve selectivity for the analytes of interest.
5. Reproducibility and Automation
Manual sample preparation can introduce variability due to operator error, leading to inconsistent results across runs. This is a significant concern for high-throughput labs.
How to Overcome:
- Automated Preparation Systems: Robotic systems can perform repetitive tasks like SPE, derivatization, and sample loading with greater precision and consistency.
- Standard Operating Procedures (SOPs): Develop clear and detailed SOPs for manual preparation to reduce variability.
- Internal Standards: Use internal standards to account for minor inconsistencies and improve reproducibility.
6. Contamination Risks
Contamination during sample preparation can result in false positives, suppressed signals, or damage to the GC system. Common sources include impure solvents, dirty glassware, or environmental factors.
How to Overcome:
- High-Purity Solvents: Always use GC-grade or HPLC-grade solvents to minimize contamination.
- Regular Equipment Maintenance: Ensure all tools, such as syringes and glassware, are thoroughly cleaned and free of residues.
- Clean Environment: Perform preparation in a contamination-controlled environment, such as a fume hood or cleanroom, to reduce external contamination.
By addressing these challenges with targeted solutions, laboratories can enhance the accuracy, sensitivity, and efficiency of their gas chromatography analyses.

Ultimately, mastering GC sample preparation is not just about following a protocol; it’s about understanding the underlying chemistry, anticipating potential interferences, and strategically applying the most appropriate techniques to achieve the desired analytical outcome. As the demands for lower detection limits, faster analysis times, and greater data integrity continue to grow, the importance of innovative and meticulous sample preparation will only intensify.
Related Products Recommendation
Get Quote Here!
Latest Posts
What Next?
For more information, or to arrange an equipment demonstration, please visit our dedicated Product Homepage or contact one of our Product Managers.







