X-ray diffraction (XRD) is widely used in metallurgy, petroleum, chemical industry, scientific research, aerospace, teaching, material production, and other fields due to its non-destructive, non-polluting, fast, high measurement accuracy, and the ability to obtain a large amount of information about crystal integrity.
As an important method for structural research, X-ray diffraction has very important applications in the research of catalytic materials and other materials. Today, this article will briefly share some basic knowledge of X-ray diffraction, so that you can have a more comprehensive understanding of this characterization method. Let’s start with practicality and let’s talk about what X-ray diffraction can do.

Does X-ray Diffraction Study the Bulk or Surface Phase of a Material?
X-ray diffraction uses monochromatic X-rays as the diffraction source, which can generally penetrate solids to verify their internal structures. Therefore, X-ray diffraction gives the bulk phase structure information of materials.
XRD Principle
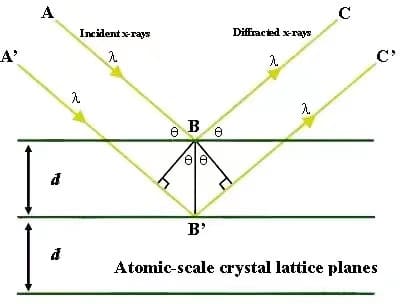
The wavelength of X-rays is similar to the spacing between the atomic planes inside the crystal, about 10-8 to 10-10 cm, so the crystal can be used as a spatial diffraction grating for X-rays. In other words, when a beam of X-rays hits an object, it is In the scattering of atoms in an object. Each atom produces scattered waves, and these waves interfere with each other, resulting in diffraction.
As a result of the superposition of diffracted waves, the intensity of the rays is strengthened in some directions and weakened in other directions. By analyzing the diffraction results, the crystal structure can be obtained. On this basis, W.L.Bragg proposed the Bragg equation as the basis of crystal diffraction:
2dsinθ=nλ
For crystal materials, when the crystal to be measured is at different angles to the incident beam, those crystal planes satisfying Bragg diffraction will be detected, which is reflected in the XRD pattern as diffraction peaks with different diffraction intensities. For amorphous materials, since there is no long-range order of atomic arrangement in the crystal structure, only a short-range order exists in the range of a few atoms, so the XRD pattern of amorphous materials shows some diffuse scattering peaks.
Is XRD a Qualitative Analysis Method or a Quantitative Analysis Method?
X-ray diffraction is mostly based on qualitative phase analysis, but quantitative analysis can also be performed.
By comparing the X-ray diffraction spectrum of the sample to be tested with the X-ray diffraction spectrum of the standard substance, the phase composition of the sample can be qualitatively analyzed.
Through the analysis and calculation of the diffraction intensity data of the sample, the quantitative analysis of the phase composition of the sample can be completed.
What Useful Information Can Be Obtained When XRD Is Used for Qualitative Analysis?
1. According to information of the X-ray diffraction spectrum, it can be determined whether the sample is amorphous or crystalline. The amorphous sample has a large package peak and no fine peak structure. The crystal has rich spectral line characteristics. Comparing the intensity of the strongest peak in the sample with that of the standard substance, the crystallinity of the sample can be known qualitatively.
2. By comparing with the standard spectrum, you can know which phases the measured sample is composed of (one of the most important uses of X-ray diffraction).
3. Through the difference between the measured sample and the standard spectrum 2θ value, it is possible to qualitatively analyze whether the unit cell expands or shrinks. The reason is that the X-ray diffraction peak position can determine the size and shape of the unit cell.
How Can X-ray Diffraction Be Used for Quantitative Analysis?
1. The average grain size of the sample
The basic principle: when the X-ray is incident on a small crystal, its diffraction lines will become diffuse and widen. The smaller the crystal grain, The wider the X-ray diffraction band big. Therefore, there is a certain relationship between the grain size and the half-width of the X-ray diffraction spectrum. That is the Scherrer equation. For the metal particles on the surface of the supported catalyst, the particle size d (unit nm) and its dispersion D can be simply converted: d ≈ 0.9/D (Note: the constant of 0.9 is an empirical value).
2. Relative crystallinity of the sample
Generally, the area (As) obtained by integrating the strongest diffraction peak is used as an indicator for calculating the crystallinity. Compared with the area (Ag) obtained by integrating the standard substance, crystallinity = As/Ag*100%.
3. Quantitative analysis of phase content
There are mainly the K value method, also called the RIR method, and Rietveld full-spectrum refined quantification. Among them, the basic principle of the RIR method is that when a substance and corundum (Al2O3) are mixed at 1:1, the integrated intensity of the strongest diffraction peak will have a ratio, which is the RIR value. The integrated intensity/RIR value of the substance can always be converted to the integrated intensity of Al2O3. For a mixture, all components in the substance are converted in this way, and finally, the percentage of a specific component can be obtained by normalization.
4. X-ray diffraction can also be used for precise calculation of lattice constants, calculation of residual stress, etc.
In conclusion, that is all about what X-ray diffraction can do, if you want to find an XRD Manufacturer, Drawell can be one of the good choices for you.



