Spectrophotometry is a powerful analytical tool used in many scientific areas to investigate the interaction of materials and electromagnetic radiation. UV-visible and infrared spectrophotometry provide unique insights into distinct elements of molecular composition. This article goes into the principles, applications, and distinctions of UV-visible spectrophotometry and Infrared spectrophotometry, emphasizing their importance in scientific research and analysis.

What are UV-visible Spectrophotometry and Infrared Spectrophotometry
UV-Visible Spectrophotometry
Principles: UV-visible spectrophotometry is the measuring of a sample’s absorption of ultraviolet (UV) and visible light. Light at various wavelengths is absorbed by molecules, triggering electronic changes inside the molecules. The resulting absorption spectrum contains useful information on the structure, concentration, and chemical environment of the substance.
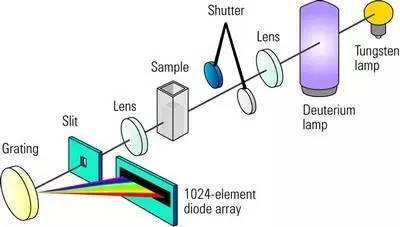
Applications: UV-visible spectrophotometry is widely used in chemistry, biochemistry, and environmental science. It is especially useful for detecting analyte concentrations in solutions, examining reaction kinetics, and investigating the electrical structure of molecules. Applications include the quantitative analysis of biomolecules such as nucleic acids and proteins, as well as the investigation of transition metal complexes.
Instrumentation: UV-visible spectrophotometers utilize a light source, a monochromator to select specific wavelengths, a sample compartment, and a detector to measure the intensity of transmitted or absorbed light. The resulting spectrum reveals characteristic peaks and troughs corresponding to electronic transitions.

Infrared Spectrophotometry
Principles: The absorption of infrared radiation by chemical bonds within a sample is the focus of infrared spectrophotometry. Specific wavelengths of infrared light are absorbed by molecules as they vibrate, bend, or spin. The infrared spectrum that results contains information about functional groups, molecule structure, and chemical bonding.
Applications: In chemistry, pharmacology, and materials research, infrared spectrophotometry is widely used. It is extremely useful for identifying and characterizing chemical compounds, analyzing polymers, and researching complicated mixtures. It aids in quality control in pharmaceutical research by recognizing certain functional groups in medication formulations.
Instrumentation: Infrared spectrophotometers consist of a light source, an interferometer to modulate the infrared beam, a sample compartment, and a detector. The resulting spectrum displays peaks corresponding to different vibrational modes, enabling the identification of specific functional groups.
Contrasts and Complementarity between UV-Visible Spectrophotometry and Infrared Spectrophotometry
1. Wavelength Range
UV-Visible Spectrophotometry
- Range: 190 to 900 nm.
- Focus: Ultraviolet and visible regions of the spectrum.
Infrared Spectrophotometry
- Range: Approximately 700 nm to 1 mm.
- Focus: Infrared region of the spectrum.
Complementarity: UV-visible and Infrared techniques cover distinct regions of the electromagnetic spectrum, enabling a comprehensive analysis of a wide range of molecular properties.
2. Information Provided
UV-Visible Spectrophotometry
- Insights: Electronic transitions, concentration.
Infrared Spectrophotometry
- Insights: Molecular vibrations, structural details.
Complementarity: UV-visible and Infrared techniques offer complementary information, allowing researchers to explore both electronic and molecular aspects of a sample’s composition.
3. Sample Types
UV-Visible Spectrophotometry
- Common Application: Analysis of samples in solution.
Infrared Spectrophotometry
- Versatility: Applicable to solids, liquids, and gases.
Complementarity: UV-visible is frequently used on solutions, whereas infrared is more adaptable and may be used on a wider range of sample types, including solids and gases.
4. Selectivity
UV-Visible Spectrophotometry
- Selective for: Compounds with conjugated pi-electron systems.
Infrared Spectrophotometry
- Sensitive to: Specific functional groups in molecules.
Complementarity: UV-visible and Infrared techniques exhibit selectivity for different molecular features, allowing researchers to choose the method that aligns with the specific characteristics of their samples.
5. Applications
UV-Visible Spectrophotometry
- Common Uses: Quantitative analysis, studying electronic structure, biomolecule analysis.
Infrared Spectrophotometry
- Common Uses: Organic compound identification, polymer analysis, studying complex mixtures.
Complementarity: UV-visible and Infrared spectrophotometry find applications in diverse scientific fields, with each excelling in areas where the other may be less suited.
6. Synergistic Application
Example: UV-visible spectrophotometry may be used by a researcher examining a complicated organic chemical to detect its concentration and electronic transitions. Simultaneously, infrared spectrophotometry can be used to detect certain functional groups and provide information on the molecular structure of the chemical.
Complementarity: Researchers can collect a more thorough dataset by combining UV-visible and Infrared spectrophotometry, offering a holistic picture of the material.

Conclusion
UV-visible and infrared spectrophotometry are essential instruments in the analytical scientist’s toolbox, each with its own set of capabilities and insights into molecular composition. Because they are complementary, researchers can use them together to gain a more comprehensive grasp of a sample’s features. Whether it’s understanding molecular vibrations in Infrared or unraveling electronic transitions in UV-visible, spectrophotometric techniques continue to drive developments in a variety of scientific fields, from pharmaceuticals to environmental science.